RG Prof. Mohrmann: Neurophysiology and cellular imaging
Research
Chemical signals are essential for neuronal communication in the nervous system. During synaptic transmission, neurotransmitter molecules are released from small synaptic vesicles, activate corresponding postsynaptic receptors, and generate electrical signals, which are in turn computed in the postsynaptic neuron. Our research group is particularly interested to understand how, the fusion of vesicles with the presynaptic membrane during exocytosis is accomplished at the molecular level. In our previous studies, we have carried out structure-function analyses on so-called SNARE proteins ("soluble NSF receptor" proteins). To this end, we have characterised the functional properties of mutant SNARE proteins using electrophysiological (membrane capacitance measurements and amperometry) and biochemical methods. We focused in particular on the SNARE protein SNAP-25, which binds two other SNARE partners and forms the central fusion complex between membranes. Our work clearly showed that the special structural properties of SNAP-25, which contains two SNARE motifs connected via a flexible linker domain, is absolutely necessary for fast and efficient membrane fusion (Shaaban et al., 2019).
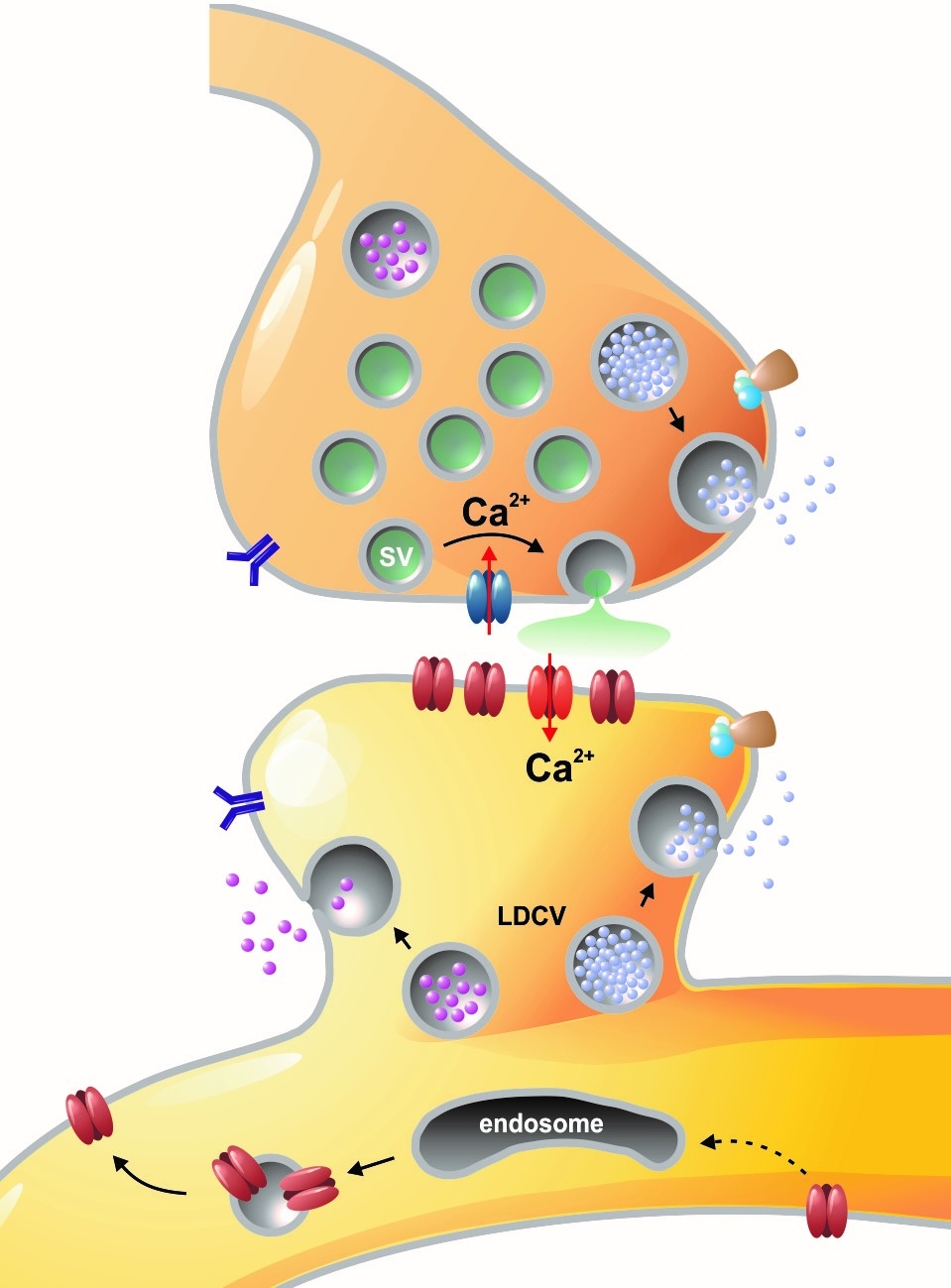
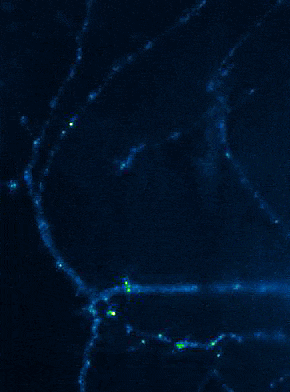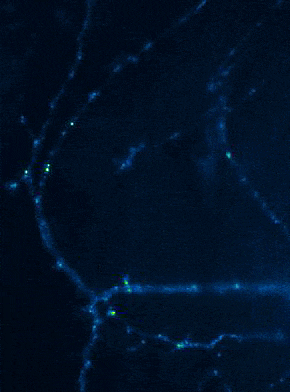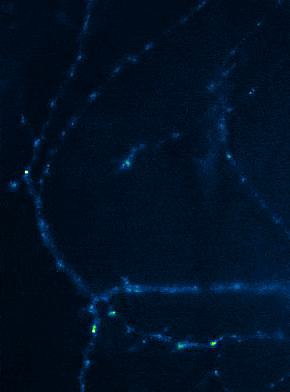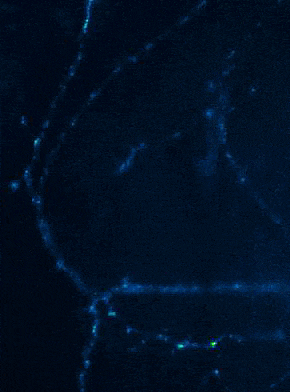
Left: Overview of membrane fusion events in the area of synapses. Right: AMPA receptor insertion events (SEP-GluR1) in dendrites.
Membrane fusion in neurons is not restricted to presynaptic boutons, but occurs during all trafficking processes between cell compartments and plasma membrane. From the perspective of neuronal information processing, it is particularly interesting to analyse the transport processes by which postsynaptic transmitter receptors reach the dendritic surface and synapses. Indeed, neurotransmitter receptors at synapses or on dendrites are subject to continuous cycles of internalisation and re-exocytosis, with receptor reinsertion usually mediated by dendritic recycling endosomes. Activity-dependent changes in the insertion rate of neurotransmitter receptors putatively underlie plastic changes in the strength of glutamatergic synapses, presumably increasing synaptic recruitment of AMPA receptors. However, the specific signals that determine whether a given AMPA receptor is subject to a rapid reinsertion cycle are still unknown. Using fluorescently labelled AMPA receptors, we therefore investigate in current research projects how the presence of different auxiliary subunits in AMPA receptor complexes affects dendritic trafficking. In an already completed study, we demonstrated in live-cell imaging experiments that the modulatory subunits Tarp-8 and CKAMP44, which are not part of the central channel pore, strongly influence the kinetics of dendritic AMPA receptor turnover. Indeed, the presence of these auxiliary subunits in AMPA receptor complexes appears to delay the internalisation-exocytosis cycle and to stabilise receptors on the cell surface (Harb et al., 2021). That said, the identification of the trafficking signals in both types of auxiliary subunits is still pending.
Methods:
- Live-cell imaging of fluorescently labelled proteins and Ca2+ imaging, confocal microscopy, 2P microscopy, immunohistochemistry.
- Electrophysiology: whole-cell patch-clamp measurements of evoked and spontaneous postsynaptic currents, membrane capacitance measurements, and amperometry.
- Molecular biology and viral expression systems
- Biochemistry: protein expression, western blots and pull-down experiments






